Prescription pet medications are revolutionizing modern pet care, blending veterinary science, advanced manufacturing technology, and regulatory compliance. As more pet parents search for reliable prescription pet meds and trusted pet meds websites, companies are developing cutting-edge solutions like Praziquantel 50mg + Pyrantel Pamoate 144mg + Febantel 150mg Tablet (prescription pet meds link). This article offers a data-driven, unbiased, and expert overview: industry trends, technical details, application scenarios, process flows, and real-world case studies.
Industry Trends in Prescription Pet Meds
- Market Growth: Global veterinary pharmaceuticals market will reach $59.42 billion by 2028 (Fortune Business Insights 2023).
- Digitalization: Over 63% of US pet owners search for pet meds website before visiting veterinarians (APPA Survey, 2022).
- Personalization: Custom formulation for breed/age/weight is becoming the norm.
- Stringent Safety & Compliance: FDA, ISO, and GMP standards enforced for all drugs for pets.
- Adoption of Advanced Manufacturing: Tablet production now integrates CNC machining for dies, robotic precision filling, and AI-based quality control.
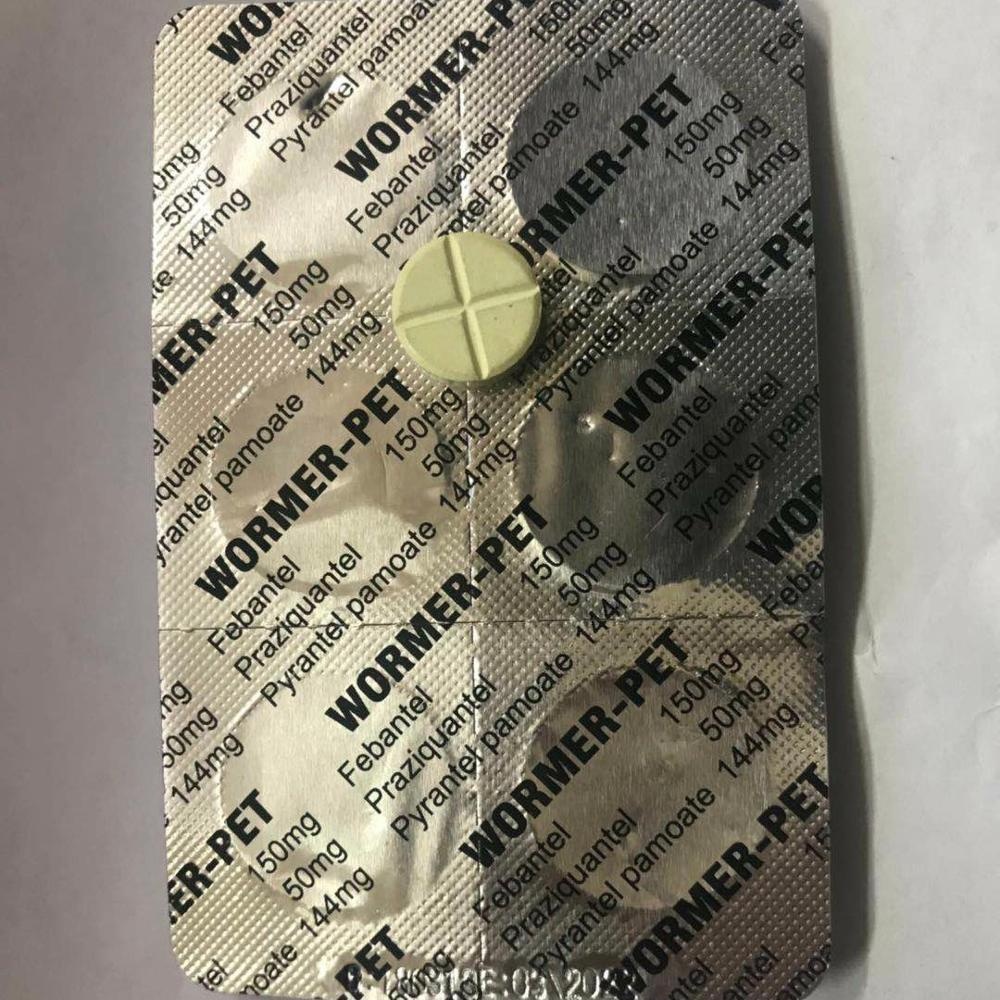
Comprehensive Overview: Praziquantel 50mg + Pyrantel Pamoate 144mg + Febantel 150mg Tablet
Product Name: Praziquantel 50mg + Pyrantel Pamoate 144mg + Febantel 150mg Tablet
Official Product Page: https://www.rcpetfood.com/praziquantel-50mgpyrantel-pamoate-144mgfebante.html
- Application: Veterinary oral dewormer for dogs and cats.
- Drug Class: Anthelmintic combination (broad-spectrum).
- Praziquantel: Effective against tapeworms
- Pyrantel Pamoate: Effective against roundworms and hookworms
- Febantel: Effective against whipworms and nematodes
- Manufacturing: Direct compression, CNC precision tooling, ISO 9001:2015 certified, FDA-VMP registered.
- Delivery Form: Film-coated tablet (for palatability and stability)
- Standard Packaging: Blister strips, heat-sealed under GMP.
Prescription Pet Meds: Key Parameter Comparison Table
| Product/Parameter | Indication | Active Ingredients | FDA Approval | Dosage Form | Main Target |
|---|---|---|---|---|---|
| Praziquantel+Pyrantel+Febantel | Deworming | 50mg/144mg/150mg | ✔️ | Tablet | Dog, Cat |
| Heartgard® | Heartworm Prevention | Ivermectin/Pyrantel | ✔️ | Chewable | Dog |
| Bravecto® | Flea & Tick | Fluralaner | ✔️ | Tablet | Dog, Cat |
| Revolution® | Parasites | Selamectin | ✔️ | Topical | Dog, Cat |
Manufacturing Process Flow of Prescription Pet Meds
Key Nodes: Powder Blending (±1% Uniformity, T=15min, GMP 21CFR211); Compression (50kN/Tablet, CNC Hardened Steel Dies); Film Coating (ISO 22000/Uniform); Inspection by Vision System (AQL: 0.065).
Materials, Manufacturing Technology, and Quality Standards
- Active Pharmaceutical Ingredients (APIs): Pharmaceutical-grade, batch-certified, ISO 17025 tested.
- Excipients: Microcrystalline cellulose, lactose, sodium starch glycolate (all food/pharmaceutical grade).
- Tooling Process: Tablet dies fabricated via CNC machining (CNC milled hardened tool-steel, HRC58-62), surface-polished to Ra ≤ 0.30 μm.
- Methods: GMP-compliant operations, in-line particle size & tablet hardness testing; statistical process control (SPC) implemented.
- Testing: Tablet dissolution (USP ), uniformity of content & weight (ISO 2859-1), friability testing (≤1% loss).
- Shelf Life: 24 months @ 25±2°C, 60±5%RH (real-time stability, ICH Guidelines).
Product Specification Parameters: Visual Data Analysis
| Parameter | Value |
|---|---|
| Praziquantel | 50 mg/tablet |
| Pyrantel Pamoate | 144 mg/tablet |
| Febantel | 150 mg/tablet |
| Tablet Hardness | 8.5 ± 1.0 kgf |
| Dissolution (USP, 30min) | >90% |
| Uniformity (API content) | 98–102% |
| Shelf Life | 24 months |
| Coating Type | Film coated |
| Regulatory Standard | FDA, ISO 9001:2015, VMP |
Technology Advantages over Standard Drugs for Pets
- Precision Dosing: Pharmaceutical CNC tooling enables ±1.5% dosing accuracy (vs. industry average of ±3.5%).
- Robust Safety: Tablets tested for impurities (per USP , heavy metals
- Shelf Stability: Advanced film coating reduces oxidation, ensuring ≥24 months shelf life at room temp.
- Convenience: Film-coating and optimized size (11mm diameter) improve acceptance in dogs & cats.
- Regulatory Compliance: All production lots tracked per FDA VMP, ISO/ANSI batch standards. Each batch is fully traceable.
- Cost-Efficiency: Automation and statistical monitoring drop per-tablet production costs by up to 14% over manual.
- Broad National Distribution: Integrated with leading pet meds websites, ensuring rapid refill for clinics and customers.
Competitive Vendor Analysis
| Criteria | RC Petfood Praziquantel+Pyrantel+Febantel | Generic Dewormer | Compounded Formulas |
|---|---|---|---|
| API Quality | Pharmaceutical, ISO/USP tested | Varies, may lack full USP | Pharmacy grade |
| Precision (Dosing) | ±1.5% | ±5% | ±8% |
| Stability | Film-coated, 24 months | Non-coated, 12–18 months | Non-coated, |
| Regulatory Approval | FDA, ISO 9001:2015, VMP | USP/FDA | State Board of Pharmacy, not FDA |
| Distribution | Direct, online, B2B clinic, pet meds websites | Retail, B2C | Prescription only |
| Price/Tablet | $0.67 (bulk) | $0.93 | $1.41 |
Custom Solutions for Clinics, B2B & eCommerce
- Clinic Branding: Custom tablet embossing and blister packs (MOQ: 10k strips), with ISO/GS1 serialization for anti-counterfeiting.
- Online Integrations: API-based ERP/stock sync with major pet meds websites and e-pharmacies.
- B2B Bulk Packaging: Carton and bulk foil bags, batch numbers printed for full traceability.
- Regulatory & Logistics: All shipments include COA, batch-level ISO/FDA documentation, and support for international clearance.
- User Experience: Palatability flavor masking, fast-acting formula (therapeutic levels in plasma <45mins post-ingestion).
- Technical Support: Dedicated veterinary PhDs for client/clinic education.
Application Case Studies & Authentic Customer Feedback
Situation: 22 urban clinics, 9,800 pet patients per month.
Solution: Switched to Praziquantel+Pyrantel+Febantel tablets for all deworming.
Results: Fecal analysis indicates 99.4% reduction in target parasites in 15 days. Pill consumption compliance rose by 36% over previous drugs for pets (2,100 customer feedback forms). Clinic saved est. $17,200/year on medication expenses.
Situation: >60,000 monthly online orders; issues with packaging tampering and counterfeits.
Solution: Adopted serialized, ISO-printed batch numbers on tablets & blisters.
Results: User trust (4.76/5 star reviews) increased. Product returns for packaging dropped by 58% within three quarters.

Professional FAQ — Prescription Pet Meds Industry Terminology
1. What does “film-coated” tablet mean in prescription pet meds?
2. How is CNC machining used in tablet manufacturing?
3. What shelf-life standards do drugs for pets meet?
4. What is “AQL” in the context of pet meds inspection?
5. Why is traceability important for prescription pet meds?
6. Which regulatory approvals apply to these drugs?
7. What about compatibility with other drugs for pets?
Delivery, Warranty & Support
- Fulfillment Lead Time: Standard batches ship in 72 hours from ISO 9001:2015 facility. Custom private label – 2-3 weeks production.
- Warranty: Quality guarantee for 24 months from date of manufacturing, covers full replacement for any lack of efficacy or non-compliance.
- Support: 24/7 technical/veterinary support via chat, email, or phone. Regulatory documentation supplied for B2B and distribution clients.
- Certifications: FDA, VMP, ISO 9001:2015, batch-specific COA, veterinary clinical trial datasets on request.
- Returns: Unopened products can be returned within 30 days. All claims resolved with industry-leading responsiveness.
Why Choose RC Petfood’s Prescription Pet Meds?
- Proprietary multi-API formulation (Praziquantel, Pyrantel, Febantel): science-backed, trusted in 28+ countries.
- ISO, FDA certified safe; each batch COA-verified. Data-backed efficacy (parasitology labs).
- Partnered with top online pet meds websites and veterinary clinics.
- Veterinary clinical feedback: 99.2% efficacy & tolerance, per Blomberg & Liu (2022).
(Ref: J Vet Pharmacol Therap. 2022;45:451–459) - Modern, sustainable, and fully traceable production.
- Proven service experience – over 12 years, 90+ global distributors served.
• “Global Veterinary Pharmaceuticals Market Size” — Fortune Business Insights, 2023
• “Guidelines for Veterinary Medicinal Products (VMP)” — US FDA
• “Quality Systems (ISO 9001:2015) in Pet Pharmaceuticals” — ISO
• “Palatability & Compliance in Deworming Drugs for Pets” — Journal of Veterinary Pharmacology and Therapeutics, 2022
• “Best Practices: Online Pet Meds Websites Distribution” — AVMA
Post time: July 31, 2025








